Adsorption Equilibrium Isotherm Study of Gold Removal Using Regenerated Activated Carbon from Cyanidation Leachate
Main Article Content
Abstract
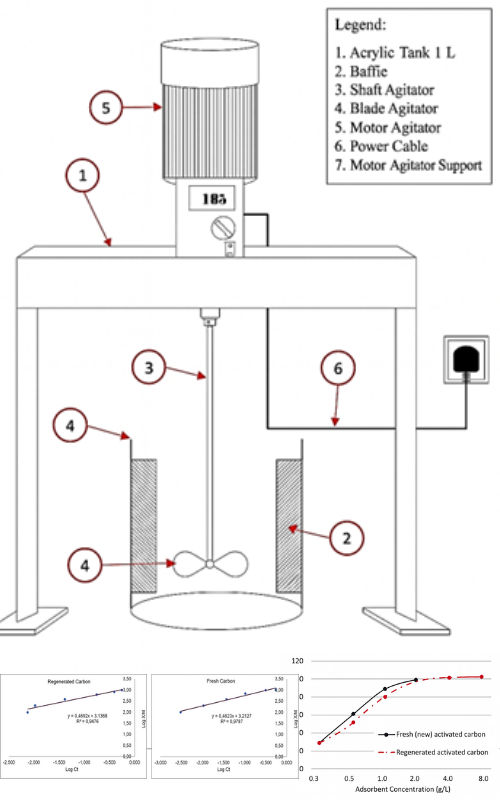
Regenerated activated carbon is a type of activated carbon that is reused in adsorption processes after undergoing elution and regeneration. This study evaluates the adsorption performance of regenerated activated carbon compared to fresh activated carbon based on the Freundlich isotherm constant and adsorption heterogeneity. Adsorption experiments were carried out on gold-bearing cyanidation leach solutions with an initial gold concentration of 0.804 mg/L using various activated carbon dosages of 0.25 g/L, 0.50 g/L, 1.0 g/L, 2.0 g/L, 4.0 g/L, and 8.0 g/L. All other parameters affecting absorptivity were kept constant for each dosage, including an adsorbate volume of 0.5 L, free cyanide concentration of 200 mg/L, agitation speed of 150 rpm, contact time of 5 hours, ambient temperature, pH of 10.5, and activated carbon particle size between 1.18-2.36 mm. The results indicate that the Freundlich adsorption constant (Kf) of regenerated activated carbon was 1370 (log Kf = 3.1368), closely comparable to that of fresh activated carbon, which was 1632 (log Kf = 3.2127). The adsorption heterogeneity index (1/n) for regenerated activated carbon was 0.47, also like that of fresh activated carbon (0.46). The optimum dosage of regenerated activated carbon was found to be 4 g/L, yielding a gold adsorption efficiency of 98.7% with a relative activity of 100% compared to fresh carbon, and resulting in a low residual gold concentration of 0.011 mg/L
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
How to Cite
References
[1] T. Syarif, A. Aladin, B. Modding, R. A. Rahmatullah, and N. M. Mustafa, “Determination of optimum pyrolysis time of coconut trunk sawdust biomass waste using argon inert gas,” Journal of Chemical Process Engineering, vol. 8, no. 1, pp. 40-48, 2023.
[2] J. Avraamides, K. Jones, W. P. Staunton, and B. Sceresini, “Gold hydrometallurgy research at the mineral processing laboratory of the Department of Mines, Western Australia,” Hydrometallurgy, vol. 30, no. 1-3, pp. 163-175, 1992, https://doi.org/10.1016/0304-386X(92)90082
[3] A. Tahad and A. S. Sanjaya, “Freundlich isotherm, kinetic model and adsorption rate of Fe using activated carbon from coffee waste,” Jurnal Chemurgy, vol. 1, no. 2, pp. 13-21, 2017, doi:https://doi.org/10.30872/cmg.v1i2.1140
[4] Norit Americas Inc., Measuring Adsorptive Capacity of Powdered Activated Carbon. Technical Bulletin, Norit Americas Inc., 2001.
[5] A. Trisnadi, Carbon Quality Comparison: PT MSM Plant Carbon and Competitor Product. Internal Technical Report, PT Meares Soputan Mining, North Sulawesi, Indonesia, Aug. 2023.
[6] S. Irfan, Studi Pengaruh Penggunaan Carbon Barren, Regenerated dan Fresh Terhadap Persen Adsorpsi dan Loading Capacity Emas-Perak pada Larutan Hasil Pelindian di PT MSM. Undergraduate thesis, UPN “Veteran” Yogyakarta, Indonesia, Oct. 2024.
[7] S. Chowdhury, R. Mishra, P. Saha, and P. Kushwaha, “Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk,” Desalination, vol. 265, no. 1-3, pp. 159-168, 2011, doi:https://doi.org/10.1016/j.desal.2010.07.047
[8] S. L. Oktaviani and N. Y. Indriyanti, “Adsorption of metal lead (Pb) in batik industrial wastewater using cellulose-based adsorbent: Literature review,” JKPK (Jurnal Kimia dan Pendidikan Kimia), vol. 7, no. 1, pp. 98-111, 2022, doi:https://doi.org/10.20961/jkpk.v7i1.55408
[9] T. S. Baunsele and C. Missa, “Langmuir and Freundlich equation test of Mn and Cu metal ion adsorption by activated carbon,” Walisongo Journal of Chemistry, vol. 4, no. 2, pp. 68-78, 2021, doi:https://doi.org/10.21580/wjc.v4i2.8539
[10] N. S. S. Miri, “Equation study of Langmuir and Freundlich isotherm on Fe(II) adsorption using zeolite and biomass-derived activated carbon,” KIREKA: Jurnal Kimia dan Rekayasa, vol. 2, no. 2, pp. 19-28, 2022.
[11] M. B. Desta, “Batch sorption experiments: Langmuir and Freundlich isotherm studies for the adsorption of textile metal ions onto teff straw (Eragrostis tef) agricultural waste,” Journal of Thermodynamics, vol. 2013, Article ID 375830, 6 pp., 2013, doi:https://doi.org/10.1155/2013/375830.
[12] R. Baby and M. Z. Hussein, “Ecofriendly approach for treatment of heavy-metal-contaminated water using activated carbon of kernel shell of oil palm,” Materials, vol. 13, no. 11, p. 2627, 2020, doi:https://doi.org/10.3390/ma13112627
[13] L. Qiu, C. Suo, N. Zhang, R. Yuan, H. Chen, and B. Zhou, “Adsorption of heavy metals by activated carbon: Effect of natural organic matter and regeneration methods of the adsorbent,” Desalination and Water Treatment, vol. 263, pp. 216-227, 2022, doi:https://doi.org/10.5004/dwt.2022.28160
[14] X. Liu, X. Xu, X. Dong, and J. Park, “Competitive adsorption of heavy metal ions from aqueous solutions onto activated carbon and agricultural waste materials,” Polish Journal of Environmental Studies, vol. 29, no. 1, pp. 749-761, 2020, doi:https://doi.org/10.15244/pjoes/104455
[15] S. Mustapha et al., “Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb(II), Cd(II), Zn(II) and Cu(II) ions from aqueous solutions using Albizia lebbeck pods,” Applied Water Science, vol. 9, no. 6, p. 142, 2019, doi:10.1007/s13201-019-1021-x
[16] R. Baby and M. Z. Hussein, “Palm kernel shell as an effective adsorbent for the treatment of heavy-metal-contaminated water,” Scientific Reports, vol. 9, p. 18955, 2019, doi:https://doi.org/10.1038/s41598-019-55099-6
[17] J. Rivera-Utrilla, M. Sánchez-Polo, V. Gómez-Serrano, P. M. Álvarez, M. C. M. Alvim-Ferraz, and J. M. Dias, “Activated carbon modifications to enhance its water treatment applications: An overview,” Journal of Hazardous Materials, vol. 187, no. 1-3, pp. 1-23, 2011, doi:https://doi.org/10.1016/j.jhazmat.2011.01.033
[18] D. Mohan and S. Chander, “Single component and multi-component adsorption of metal ions by activated carbons,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 177, no. 1, pp. 183-196, 2001, doi:https://doi.org/10.1016/S0927-7757(00)00687-X
[19] M. Franz, H. A. Arafat, and N. G. Pinto, “Effect of chemical surface heterogeneity on the adsorption mechanism of dissolved aromatics on activated carbon,” Carbon, vol. 38, no. 13, pp. 1807-1819, 2000, doi:https://doi.org/10.1016/S0008-6223(00)00064-1
[20] J. Rivera-Utrilla and M. Sánchez-Polo, “Adsorption of Cr(III) on ozonised activated carbon: Importance of Cπ-cation interactions,” Water Research, vol. 37, no. 13, pp. 3335-3340, 2003, doi:https://doi.org/10.1016/S0043-1354(03)00233-5
[21] R. Tarigan, “Adsorption mechanism of heavy metals using activated carbon,” Thai Industrial and Systems Engineering Journal, vol. 19, no. 1, pp. 31-45, 2025.
[22] M. Arman, Z. Sabara, and T. Arief, “The Effect of Pyrolysis Temperature on Sawdust-Biomass Activated Carbon Using NaOH and NaCl Activators”, Eng. J., vol. 28, no. 8, pp. 1-11, Aug. 2024. DOI: https://doi.org/10.1016/S0043-1354(03)00233-5
[23] R. Baby, M. Z. Hussein, and co-authors, “Preparation of functionalized palm kernel shell bio-adsorbent for the removal of heavy metals from aqueous solution,” Environmental Advances, vol. 10, p. 100262, 2023, doi:https://doi.org/10.1016/j.envadv.2023.100262
[24] T. Ahmad, M. Ismail, and S. H. Marsin, “Recent advances in the preparation of oil palm waste-based adsorbents for removal of environmental pollutants: A review,” Malaysian Journal of Analytical Sciences, vol. 22, no. 2, pp. 175-184, 2018, doi:https://doi.org/10.17576/mjas-2018-2202-02.
[25] M. Gayathiri et al., “Sustainable oil palm trunk fibre-based activated carbon for adsorption of methylene blue dye,” Scientific Reports, vol. 13, no. 1, p. 49079, 2023, doi:https://doi.org/10.1038/s41598-023-49079-0