Effect of Sulfuric Acid Concentration on the Leaching Behavior of Manganese Ore from Palludda Barru Regency
Main Article Content
Abstract
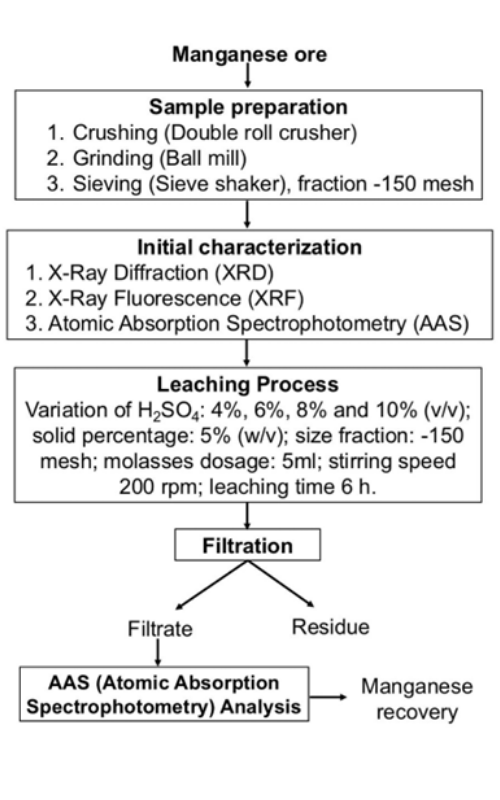
Manganese is one of the minerals that constitute various rock formations found throughout Indonesia, particularly in the Sulawesi region. Therefore, research on the processing of manganese ore is essential to ensure its availability can be utilized efficiently and effectively. The objective of this study is to determine the effect of varying H₂SO₄ concentrations and identify the optimum concentration for achieving the highest recovery in manganese ore processing. One method to enhance manganese ore grade is by applying a leaching process using H₂SO₄ solution with concentration variations of 4%, 6%, 8%, and 10%, with a solid mass of 5 grams for each concentration. The leaching process was carried out for 6 hours at each concentration with a stirring speed of 200 rpm. The resulting filtrate was analyzed using Atomic Absorption Spectroscopy (AAS). The leaching results yielded dissolved Mn with 47.600% at 4% concentration, 52.585% at 6%, 50.141% at 8%, and 50.557% at 10%. Based on the results, the highest Mn extraction was achieved at a 6% concentration with a recovery of 52.585% after 6 hours of leaching.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.